Why need viral vector characterization?
Cell and gene therapies for various rare diseases are currently undergoing clinical trials worldwide. The rapid development in this field has led to an increase in regulatory scrutiny and product characterization requirements, as well as a bottleneck in viral vector supply. The manufacturing processes and analytical tools for gene therapy viral vectors need to be continuously improved to meet these demands. Viral vector characterization covers a wide range of viral vectors, such as lentiviral (LV) vectors, adenoviral vectors, and adeno-associated viral (AAV) vectors. Sustained improvements and the establishment of rapid and robust viral vector characterization techniques are crucial for stringent quality control, in order to increase the likelihood of successful development of the required gene therapies.
The high complexity of viral vectors is one of the challenges in their characterization and quality control testing. Establishing accurate, fast, and reproducible analytical methods to provide researchers with data that is closer to real-time is the goal. These data can offer important information for decision-making in the development of virus-based gene therapies, enabling the production of high-quality, safe, consistent, and effective products.
Viral vector manufacturing
During process development/optimization, the FDA mandates that overall product characterization under cGMP (current Good Manufacturing Practice) follows SISPQ (Safety, Identity, Strength/Potency, Purity, and Quality).
l Safety: The product does not elicit unexpected side effects when used appropriately in the patient.
l Identity: The product is exactly what is described on the label.
l Strength/Potency: The product delivers the correct dosage over the shelf-life of the product.
l Purity: The product is free from physical, biological, and chemical contamination.
l Quality: The product is manufactured using established quality systems to ensure product consistency and quality specifications are met.
(1) Quantification of viral vectors: confirming the identity of viral vectors and quantifying viral particles during growth, purification, final quality control, and release.
(2) Evaluation of viral vector purity: detect and quantify impurities derived from the host cell system where the vector product is produced or from downstream purification processes using sensitive and ready-to-use methods.
(3) Capsid analysis of viral vectors: capsid analysis of viral vectors, including purity analysis, full/empty capsid ratio determination, genome integrity analysis, and characterization of capsid proteins.
(4) Biosafety testing of viral vectors: biosafety assays for bacteria, fungi, mycoplasma, and exogenous viruses on drug materials to ensure the identity, efficacy, purity, and safety of gene therapies developed for diseases.
(5) Stability testing of viral vectors: using a variety of technologies to test for multiple physical parameters such as pH/osmotic pressure or the presence of protein aggregates to ensure that quality properties are maintained.
(6) Evaluation of targeting capabilities of viral vectors: assess the targeting capabilities and biodistribution analysis of viral vectors through model systems and imaging technologies.
In summary, viral vector characterization offers a comprehensive understanding of the physical properties of viral vectors, including identity, purity, strength/potency, integrity, safety, and stability, throughout the gene therapy development process, from early development to full-scale manufacturing.




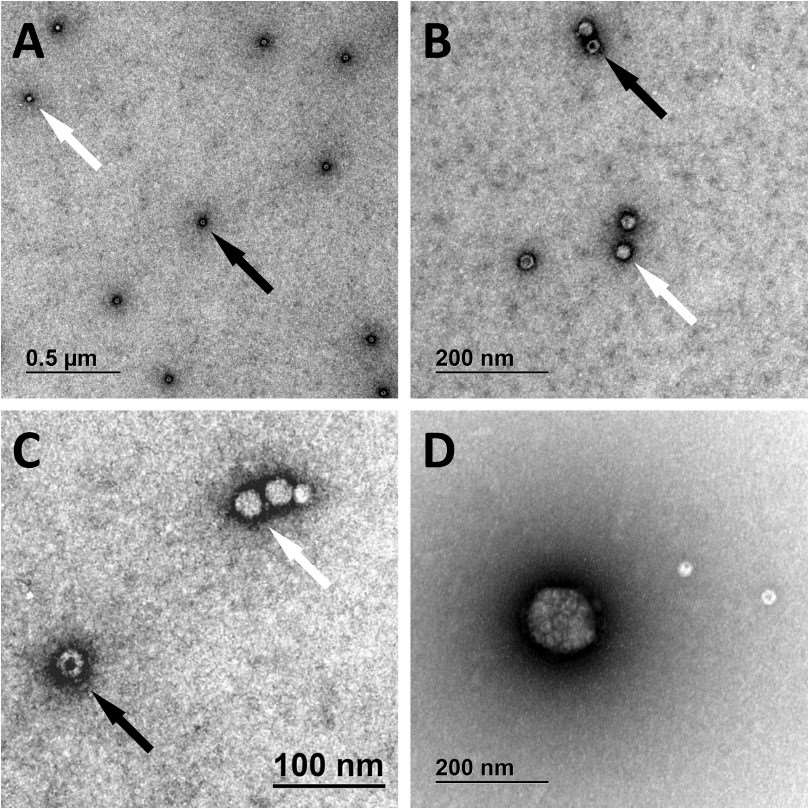
Comments