As several gene therapy approvals for rare disease treatment gain steam in 2023, a comprehensive set of gene therapy development solutions is released to pave richer avenues for rare disease research. This path-breaking move sees the integration of cutting-edge technology with state-of-art research facilities to amplify the possibilities of devising effective remedies for rare diseases.
Rare diseases, despite their name, affect nearly 350 million people worldwide. However, due to their scarcity and diversity, research in this area has been traditionally challenging, making this development a beacon of hope for patients and researchers worldwide. After decades of intense scientific research into developing gene editing technologies, despite several obstacles, gene therapy for rare diseases has fostered several successful instances recently. The year 2023 has witnessed several FDA approvals so far, each marking the first time gene therapy has been approved for different rare diseases. These achievements indicate a growing inclination among regulatory bodies to accept data on safety, efficacy, and durability for the endorsement of these treatments.
Recognizing the dire need for accelerated research in the field of rare diseases, the comprehensive gene therapy development solutions aim to overhaul the traditional slow and resource-intensive process of genetic research. The use of directed evolution, high throughput screening platforms, and vector production will boost the adoption and efficiency of gene therapy, thereby revolutionizing research methodology.
Gene therapy development solutions, including viral and non-viral vector development, gene editing technology support, and gene therapy safety enhancement, are provided for innovative biotechnology companies, research institutions, and pharmaceutical companies to accelerate their development of safer and more effective gene therapies for rare diseases and enhance the speed and caliber of research in various strains of rare diseases. From the target gene selection and preparation to gene editing and final evolution, clients’ needs and requirements will be fully considered and followed.
About rare disease gene therapy
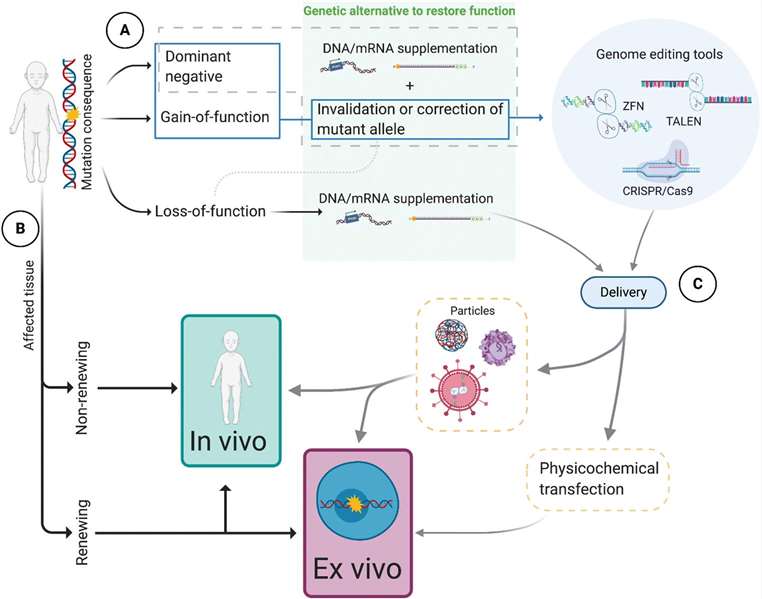
More than 80% of rare diseases are single-gene genetic disorders and lack of effective treatments. Gene therapy can alter the biology of living cells by modifying or manipulating gene expression, offering the possibility of correcting underlying genetic defects to address the underlying cause of rare diseases. With the development of genetic diagnostics for rare diseases, advances in vector delivery technologies and biotechnologies such as CRISPR-Cas9, gene therapy has become one of the key research directions for therapeutic drugs for rare diseases.




Comments