Roots Analysis has done a detailed report on Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA, covering key aspects of the industry and identifying key future growth opportunities.
Key Market Insights
§ Presently, more than 55 automatic sampling systems are commercially available in different regions of the globe; a relatively larger proportion of these players are mid-sized companies
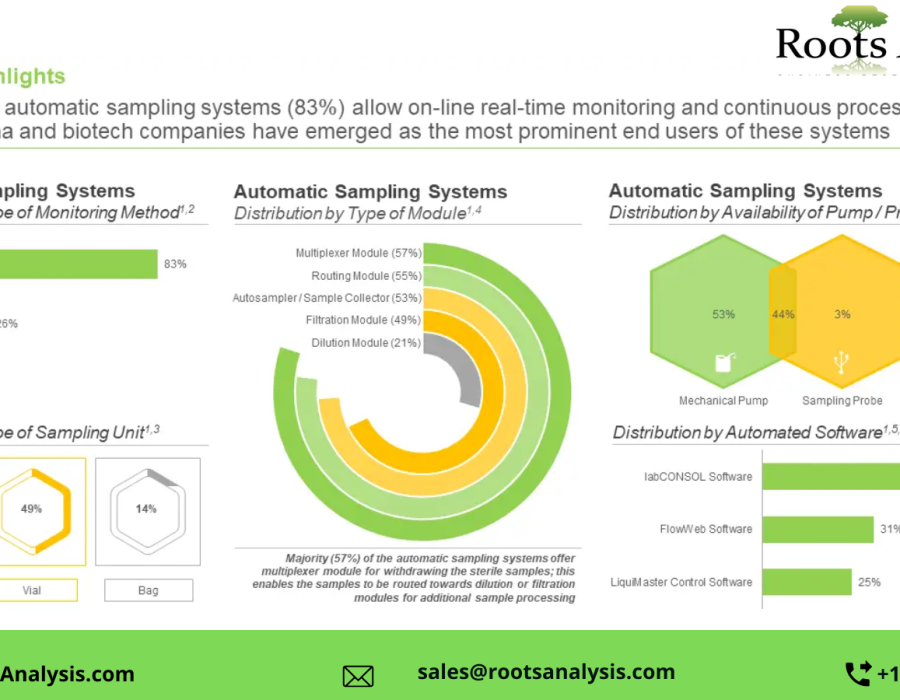
§ Majority of the automatic sampling systems (83%) allow on-line real-time monitoring and continuous process control; pharma and biotech companies have emerged as the most prominent end users of these systems
§ More than 80% of the automatic sample collection / preparation system manufacturers were established before 2000; majority of them are based in North America
§ In pursuit of building a competitive edge, stakeholders in this domain are striving to enhance their product portfolio and upgrade their automatic sampling systems with advanced features
§ Over 400 patents related to automatic sampling in biopharmaceutical industry have been filed / granted by various stakeholders in order to protect the intellectual property generated within this field
§ The growing interest is also reflected by the events organized for automatic sampling systems, providing an opportunity to product developers to share their ideas and develop a better understanding of such systems
§ Over 35% of the partnerships were signed for product distribution purposes; stakeholders based in North America have signed maximum agreements related to automatic sampling
§ The increasing adoption of automatic sampling systems in the biopharmaceutical industry is anticipated to create profitable business opportunities for the system manufacturers
§ Given the requirement of systems that can transfer bioprocess samples directly from bioreactors to analyzers while maintaining the process sterility, the adoption of automatic sampling is anticipated to rise significantly
§ The market is likely to grow at an annualized rate of more than 15% by 2035; the opportunity is likely to be well distributed across different geographies, and other important market segments
Table of Contents
1. PREFACE
1.1. Scope of the Report
1.2. Market Segmentation
1.3. Research Methodology
1.4. Key Questions Answered
1.5. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
3.1. Chapter Overview
3.2. Process Analytical Technology in Sampling
3.3. Manual Sampling versus Automatic Sampling
3.4. Need for Automatic Sampling Systems
3.5. Automatic Aseptic Sampling System
3.6. Components of a Sampling System
3.7. Stand-alone Systems versus Integrated Systems
3.8. Bioprocess Monitoring and Control Methods
3.8.1. At-line Monitoring
3.8.2. In-line Monitoring
3.8.3. Off-line Monitoring
3.8.4. On-line Monitoring
3.9. Key Considerations for Automatic Sampling
3.9.1. Sample Volume
3.9.2. Cell Removal
3.9.3. Sampling Rate
3.9.4. Integration of Analyzers
3.9.5. Feedback to Bioreactor
3.9.6. Flexibility
3.9.7. Transferability
3.9.8. Price
3.10. Benefits of Automatic Sampling Systems
3.11. Standards and Requirements
3.12. Future Innovations
4. MARKET LANDSCAPE: AUTOMATIC SAMPLING SYSTEMS
4.1. Chapter Overview
4.2. Automatic Sampling Systems: Overall Market Landscape
4.2.1. Analysis by Type of Monitoring Method
4.2.2. Analysis by Type of Sampling Unit
4.2.3. Analysis by Availability of Pump / Probe
4.2.4. Analysis by Type of Module
4.2.5. Analysis by Automated Software
4.2.6. Analysis by Type of Vessel
4.2.7. Analysis by Vessel Fabrication Material
4.2.8. Analysis by Type of Analyte Monitored
4.2.9. Analysis by Type of Analyzer
4.2.10. Analysis by Number of Sampling Vessels
4.2.11. Analysis by Working Volume
4.2.12. Analysis by Operating Temperature
4.2.13. Analysis by End User Industry
4.2.14. Analysis by Scalability
4.2.15. Analysis by Application(s)
4.3. List of Automatic Sampling System Manufacturers
4.3.1. Analysis by Year of Establishment
4.3.2. Analysis by Company Size
4.3.3. Analysis by Region of Headquarters
4.3.4. Analysis by Company Size and Region of Headquarters
4.3.5. Analysis by Location of Headquarters
4.3.6. Leading Players: Analysis by Number of Automatic Sampling Systems Manufactured
4.2.7. Leading Automatic Sampling System Manufacturers: Analysis by Number of End User Industries
5. COMPANY COMPETITIVENESS ANALYSIS
5.1. Chapter Overview
5.2. Assumptions / Key Parameters
5.3. Scope and Methodology
5.4. Company Competitiveness Analysis: Automatic Sampling System Manufacturers in North America
5.5. Company Competitiveness Analysis: Automatic Sampling System Manufacturers in Europe
5.6. Company Competitiveness Analysis: Automatic Sampling System Manufacturers in Asia-Pacific
6. COMPANY PROFILES: AUTOMATIC SAMPLING SYSTEM MANUFACTURERS
6.1. Chapter Overview
6.2. Agilent Technologies
6.2.1. Company Overview
6.2.2. Financial Information
6.2.3. Product Portfolio
6.2.4. Recent Developments and Future Outlook
6.3. Cytiva
6.3.1. Company Overview
6.3.2. Product Portfolio
6.3.3. Recent Developments and Future Outlook
6.4. Mettler Toledo
6.4.1. Company Overview
6.4.2. Financial Information
6.4.3. Product Portfolio
6.4.4. Recent Developments and Future Outlook
6.5. Pall Corporation
6.5.1. Company Overview
6.5.2. Product Portfolio
6.5.3. Recent Developments and Future Outlook
6.6. Shimadzu
6.6.1. Company Overview
6.6.2. Financial Information
6.6.3. Product Portfolio
6.6.4. Recent Developments and Future Outlook
6.7. Xylem
6.7.1. Company Overview
6.7.2. Financial Information
6.7.3. Product Portfolio
6.7.4. Recent Developments and Future Outlook
7. MARKET LANDSCAPE: AUTOMATIC SAMPLE COLLECTION / PREPARATION SYSTEMS
7.1. Chapter Overview
7.2. Automatic Sample Collection / Preparation Systems: Overall Market Landscape
7.2.1. Analysis by System Category
7.2.2. Analysis by System Classification
7.2.3. Analysis by Type of Monitoring Method
7.2.4. Analysis by Type of Sampling Unit
7.2.5. Analysis by Type of Module
7.2.6. Analysis by Working Volume
7.2.7. Analysis by Type of Analyzer
7.2.8. Analysis by End User Industry
7.2.9. Analysis by Scalability
7.3. List of Automatic Sample Collection / Preparation System Manufacturers
7.3.1. Analysis by Year of Establishment
7.3.2. Analysis by Company Size
7.3.3. Analysis by Region of Headquarters
7.3.4. Analysis by Company Size and Region of Headquarters
7.3.5. Analysis by Location of Headquarters
7.3.6. Leading Players: Analysis by Number of Automatic Sample Collection / Preparation Systems Manufactured
8. COMPANY PROFILES: AUTOMATIC SAMPLE COLLECTION / PREPARATION SYSTEM MANUFACTURERS
8.1. Chapter Overview
8.2. Agilent Technologies
8.2.1. Company Overview
8.2.2. Product Portfolio
8.2.3. Recent Developments and Future Outlook
8.3. Biotage
8.3.1. Company Overview
8.3.2. Product Portfolio
8.3.3. Recent Developments and Future Outlook
8.4. Flownamics
8.4.1. Company Overview
8.4.2. Product Portfolio
8.4.3. Recent Developments and Future Outlook
8.5. MGI Tech
8.5.1. Company Overview
8.5.2. Product Portfolio
8.5.3. Recent Developments and Future Outlook
8.6. SOTAX
8.6.1. Company Overview
8.6.2. Product Portfolio
8.6.3. Recent Developments and Future Outlook
9. PATENT ANALYSIS
9.1. Chapter Overview
9.2. Scope and Methodology
9.3. Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA: Patent Analysis
9.3.1. Analysis by Publication Year
9.3.2. Analysis by Annual Number of Granted Patents and Patent Applications
9.3.3. Analysis by Geographical Location
9.3.4. Analysis by CPC Symbols
9.3.5. Word Cloud: Emerging Focus Areas
9.3.6. Analysis by Type of Organization
9.3.7. Leading Industry Players: Analysis by Number of Patents
9.3.8. Leading Non-Industry Players: Analysis by Number of Patents
9.3.9. Leading Individual Assignees: Analysis by Number of Patents
9.4. Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA: Patent Benchmarking Analysis
9.4.1. Analysis by Patent Characteristics
9.5. Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA: Patent Valuation Analysis
9.6. Leading Patents by Number of Citations
10. RECENT DEVELOPMENTS
10.1. Chapter Overview
10.2. Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA: Partnership Activity
10.2.1. Partnership Models
10.2.2. List of Partnerships and Collaborations
10.2.2.1. Analysis by Year of Partnership
10.2.2.2. Analysis by Type of Partnership
10.2.2.3. Analysis by Type of Product
10.2.2.4. Analysis by Product and Type of Partnership
10.2.2.5. Most Active Players: Analysis by Number of Partnerships
10.2.2.6. Word Cloud: Emerging Focus Areas
10.2.2.7. Regional Analysis
10.2.2.8. Intercontinental and Intracontinental Agreements
10.3. Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA: Global Events
10.3.1. List of Global Events
10.3.1.1. Analysis by Year of Event
10.3.1.2. Analysis by Event Platform
10.3.1.3. Analysis by Type of Event
10.3.1.4. Analysis by Geography
10.3.1.5. Word Cloud: Evolutionary Trends in Event Agenda / Key Focus Area
10.3.1.6. Most Active Participants: Analysis by Number of Events
10.3.1.7. Analysis by Seniority Level of Event Speakers
10.4. Concluding Remarks
11. SWOT ANALYSIS
11.1. Chapter Overview
11.2. Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA: SWOT Analysis
11.2.1. Comparison of SWOT Factors
12. PORTER’S FIVE FORCES ANALYSIS
12.1. Chapter Overview
12.2. Methodology and Assumptions
12.3. Key Parameters
12.3.1. Threats of New Entrants
12.3.2. Bargaining Power of Customers
12.3.3. Bargaining Power of Automatic Sampling System Manufacturers
12.3.4. Threats of Substitute Products
12.3.5. Rivalry Among Existing Competitors
12.4. Concluding Remarks
13. MARKET FORECAST AND OPPORTUNITY ANALYSIS
13.1. Chapter Overview
13.2. Key Assumptions and Methodology
13.3. Global Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA Market, 2022-2035
13.3.1. Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA Market, 2022 and 2035: Distribution by Type of Monitoring Method
13.3.1.1. Automatic Sampling Market for On-line Monitoring, 2022-2035
13.3.1.2. Automatic Sampling Market for Off-line Monitoring, 2022-2035
13.3.1.3. Automatic Sampling Market for At-line Monitoring, 2022-2035
13.3.2. Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA Market, 2022 and 2035: Distribution by Type of Bioprocessing Method
13.3.2.1. Automatic Sampling Market for Upstream Bioprocessing, 2022-2035
13.3.2.2. Automatic Sampling Market for Downstream Bioprocessing, 2022-2035
13.3.3. Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA Market, 2022 and 2035: Distribution by Working Volume
13.3.3.1. Automatic Sampling Market for Systems with less than 10 mL Working Volume, 2022-2035
13.3.3.2. Automatic Sampling Market for Systems with 10-50 mL Working Volume, 2022-2035
13.3.3.3. Automatic Sampling Market for Systems with 51-100 mL Working Volume, 2022-2035
13.3.3.4. Automatic Sampling Market for Systems with more than 100 mL Working Volume, 2022-2035
13.3.4. Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA Market, 2022 and 2035: Distribution by Scalability
13.3.4.1. Automatic Sampling Market for Lab Scale Operations, 2022-2035
13.3.4.2. Automatic Sampling Market for Pilot Scale Operations, 2022-2035
13.3.4.3. Automatic Sampling Market for Commercial Scale Operations, 2022 2035
13.3.5. Automatic Sampling in Biopharmaceutical Applications and the Measurement of CQA Market, 2022 and 2035: Distribution by Key Geographical Regions
13.3.5.1. Automatic Sampling Market in North America, 2022-2035
13.3.5.2. Automatic Sampling Market in Europe, 2022-2035
13.3.5.3. Automatic Sampling Market in Asia-Pacific and Rest of the World, 2022 2035
14. INDUSTRIAL REVOLUTION FROM INDUSTRY 1.0 TO INDUSTRY 5.0
14.1. Chapter Overview
14.2. Transition from Industry 1.0 to Industry 5.0
14.2.1. Industry 1.0
14.2.2. Industry 2.0
14.2.3. Industry 3.0
14.2.4. Industry 4.0
14.2.5. Industry 5.0
14.3. Horizons of Lab Evolution
14.4. Benefits of Industry 4.0
14.5. Benefits of Industry 5.0
14.6. Concluding Remarks
15. CONCLUDING REMARKS
16. EXECUTIVE INSIGHTS
17. APPENDIX 1: TABULATED DATA
18. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
To view more details on this report, click on the link:
https://www.rootsanalysis.com/reports/automatic-sampling-market.html
You may also be interested in the following titles:
Decentralized Clinical Trials / Virtual Clinical Trials Market
You may also like to learn what our experts are sharing in Roots educational series:
4D Bioprinting Market: Key Trends
Applications of Blockchain in Various Sectors: An Introduction
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091




Comments