Scope
The document outlines criteria for symbols utilized in medical device labeling to communicate information regarding their safe and efficient usage. It additionally enumerates symbols that meet these criteria. This guideline pertains to symbols employed across various medical devices sold worldwide, necessitating compliance with diverse regulatory standards. These symbols may feature on the medical device, its packaging, or accompanying documentation. However, it’s important to note that this document’s specifications do not extend to symbols outlined in alternate standards.

Background Of ISO 15223
The history behind ISO 15223 is extensive, having developed through collaboration among industry specialists and stakeholders. Its publication aimed to establish a universally comprehensible set of symbols and labeling standards. ISO 15223 has achieved widespread recognition and implementation within the medical device industry on a global scale.
Significance Of Symbol
Symbols are visual representations found on the label or accompanying documents of a medical device. They convey important information without requiring the reader to understand a specific language of any nation or culture.
Benefits And Impact
The adoption of ISO 15223 yields concrete advantages. Enhanced patient safety, simplified global commerce, and better communication between stakeholders are among the notable outcomes. We’ll explore case studies and practical instances illustrating the significant influence of following this standard.
Amendments Of Guidelines

Commonly Used Symbols On Medical Device
Frequently utilized symbols found on medical devices serve to provide users with general information about the device and to display its unique identification number.
General Symbols
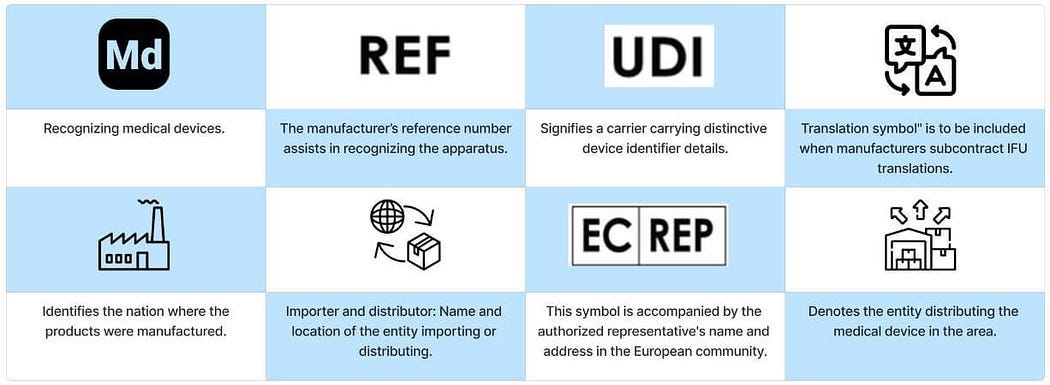
Manufacturer Symbols

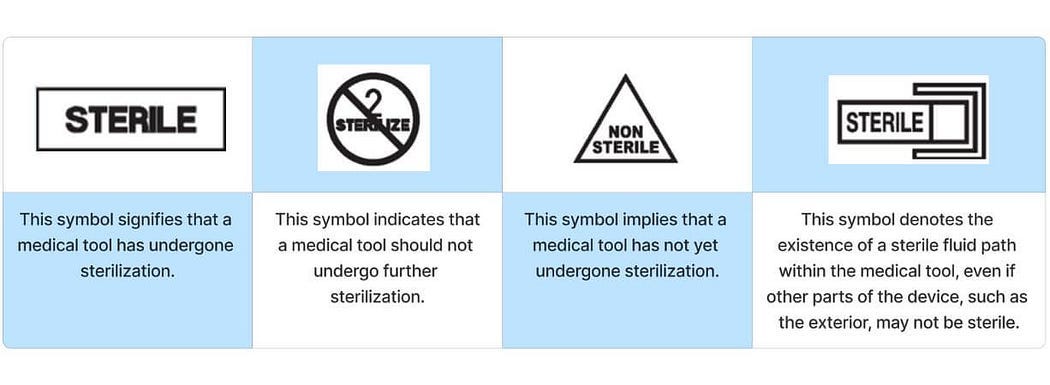
Sterile Symbols
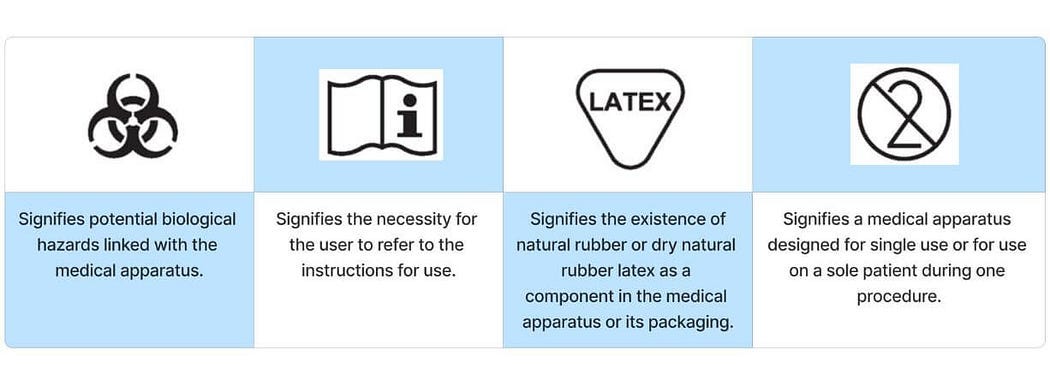
Safety Symbol
Storage symbol

Country specific

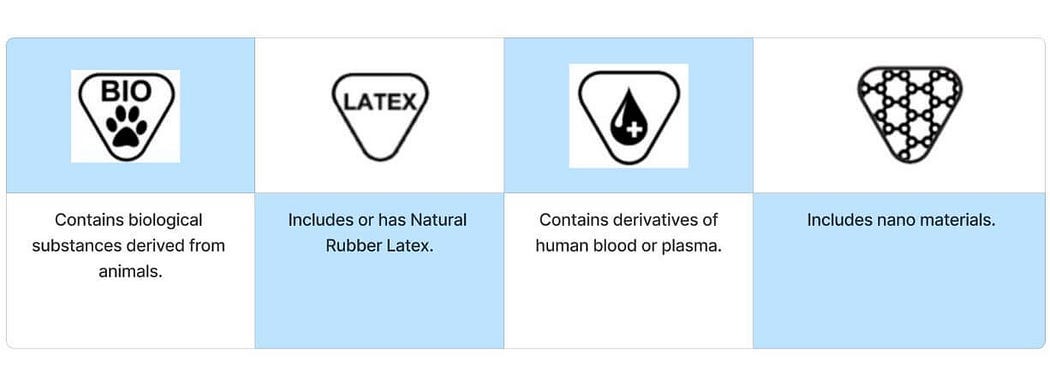
Materials substances
References
International standard ISO — 15223 -1:2021
For more details visit MAVEN…





Comments